Solid state, matter, corrosion and pitting corrosion: these are just some of the words that our blog is full of. Technical concepts to which not all the operators of the field are accustomed.
To make it easier to understand, our expert will give us some small introductory articles on these subjects.
Let’s start from the basis: The matter.
The term matter, in classical physics, is used to define any object having a mass and occupying a space. We have specified “classical physics” because in modern physics, this definition would not be considered complete.
Everything that has mass and occupies space, therefore, is considered matter.
This is divided into homogeneous and heterogeneous. The distinction between these two types is based on the number of phases (that is the number of physically distinguishable and delimited portions) which compose them.
Homogeneous → one phase ⇒ es. Air (each section has the same composition)
Heterogeneous → more phases ⇒ es. Fog (each section consists of air + water)
What is it composed of?
Determining the type of matter also helps us to know its structure at a microscopic level. Matter, in fact, is made up of atoms and these, in turn, are made up of small particles called protons, neutrons and electrons. The arrangement of these atoms makes the various substances distinguishable by type of state.
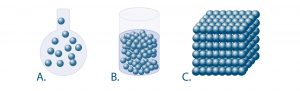
The main types of states (picture below) are:
- Gaseous state (picture A.)
- Liquid state (picture B.)
- Solid state (picture c.)
As for the stainless steel field, what interests us most is the solid state.
Solid state.
The existence of interatomic forces of a certain importance causes atoms to naturally aggregate in compact structures.
The solid state can be described by a geometric model according to which atoms are assimilated to hard and impenetrable spheres.
The size of the spheres, or atoms, is linked to the type of element and to the distance of the bond between the atoms.
A homogeneous arrangement allows a smooth and durable structure. The placement of hard spheres in space, however, does not always occur in a regular manner. Very often the conditions of temperature, pressure, chemical environment (in which materials are formed) do not allow the most effective arrangement to be reached, on the contrary, they promote the creation of defects in the structures.
The arrangement of hard spheres in space, therefore, can give rise to more or less dense configurations.
The coordinator number.
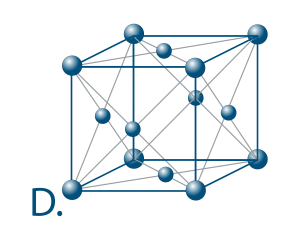
The number of atoms that surround an atom in a structure closely is called the coordination number. It indicates how effectively and tightly the atoms are compressed.
The maximum coordination number for a solid is 12. It is found in the centred cubic lattices (picture D.) which is not by chance the austenitic stainless steels of which we will speak later.
See you on next Expert Lesson.
Bolt rims, fittings, nautical stainless steel accessories or fasteners for photovoltaic systems? Visit our website and register at our e-commerce to view in real time prices and availability of all our products.
Click here to read other articles of our blog!